LET’S LOVE
EVERY
BODY


Inclusive of many kinds of bodies
Estrogen-free Slynd offers a manageable bleeding profilea with ¹ flexible 24-hour missed pill window. ²
¹ In clinical studies, 3.5% of patients discontinued due to bleeding irregularities.
² Tablets must be taken every day at about the same time of the day so that the
interval between two tablets is always 24 hours.
Efficacy
Slynd provides effective pregnancy prevention…ª,³
4.0
Pearl index
(95% CI; 2.3, 6.4)
out of 5547 evaluable cycles
98.2%
Avoided pregnancy in clinical studies1 1.8% became pregnant
17 out of 953 females evaluated
332
High BMI women included in clinical trial studies ¹,º
Mean BMI 28.5 kg/m2
332 subjects BMI ≥ 30 (35%)
173 females had BMI ≥ 35 (18%)
c Efficacy was assessed in a single arm trial of 953 women 35 years or younger who were of reproductive potential.
º The data was insufficient to analyze Pearl Index by BMI subgroups.
Slynd has proven in clinical studies with over 3,400 women that it is a safe oral contraceptive² and offers a convenient option for many telemedicine patients.
Slynd does not require a blood pressure check prior to initiation.
No boxed warning!

Unlike estrogen-containing products, there is no evidence of increased risk of myocardial infarction, cerebral thromboembolism, or venous thromboembolism reported in epidemiological studies for progestin-only products.

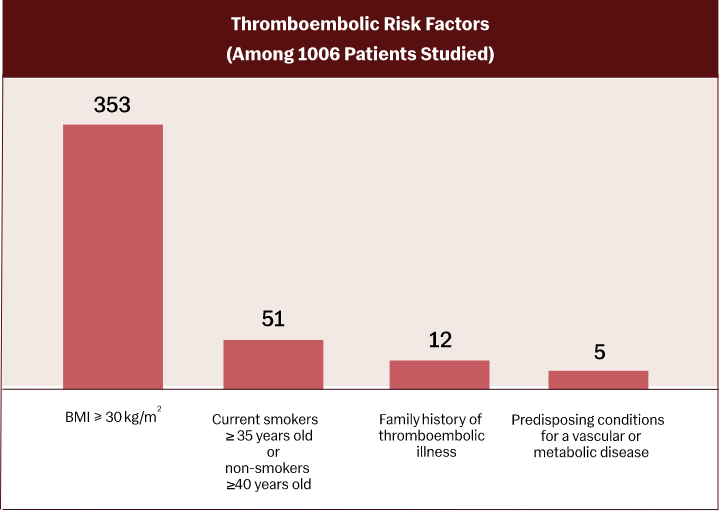
The Slynd pivotal trial included 39.2% of patients with 1 or 2 VTE risk factors¹,²:
Features of Slynd
Missed pill window
Slynd has a flexible 24-hour missed pill
window1,e. This provides the window she needs to take Slynd effectively.

Slynd’s 30-hour elimination half-life helps maintain therapeutic plasma levels.1
e Slynd tablets must be taken every day at the same time of the day. If one active tablet is missed, patients must take the missed tablet as soon as possible.

Slynd 24+4 dosing regimen
Slynd has a familiar 24+4 dosing regimen that is similar to COCs2.
f Brand name Micronor.
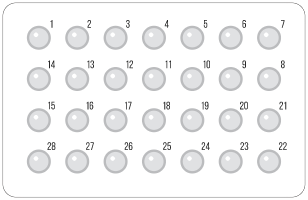
Norethindrone POPs 3,f
28 pill dosing regimen
No placebo pills
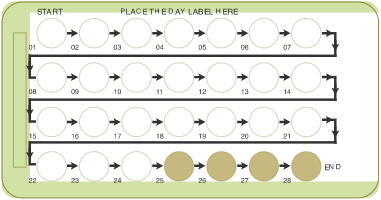
Slynd
24+4 dosing regimen
24 active hormone pills + 4 placebo pills
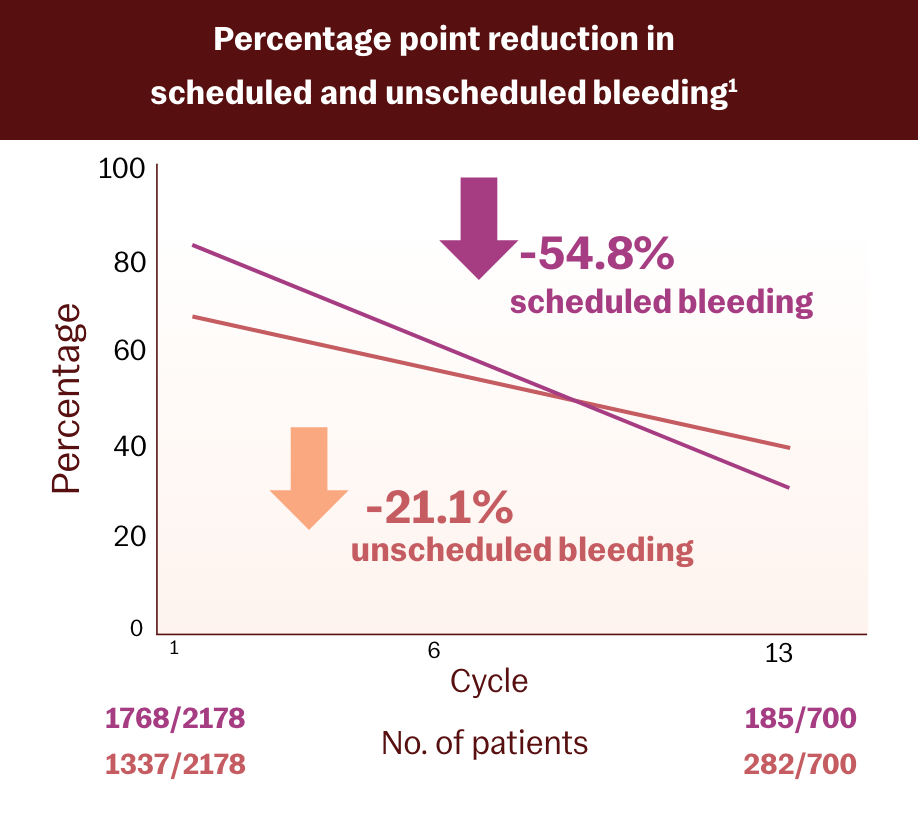
Slynd has a manageable bleeding profileg, h, i
In clinical studies, over 13 cycles:
- Breakthrough bleeding improved over time1
- At cycle 12, 50.6% of subjects reported amenorrhea2,g
- Discontinuation rate due to bleeding irregularities was 3.5% of total subjects2,h
g In clinical studies, 3.5% of patients discontinued due to bleeding irregularities.
h No bleeding or spotting days reported during entire exposure cycle.
i A total of 91 out of 2593 subjects discontinued Slynd due to menstrual bleeding disorders including metrorrhagia, menstrual irregular, vaginal hemorrhage, menorrhagia, uterine hemorrhage, and amenorrhea.
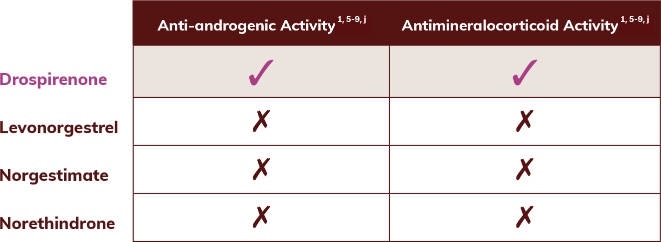
The active ingredient in Slynd is drospirenone1
Drospirenone features include anti-androgenic and antimineralocorticoid activity1
- Has no known androgenic activity
- Associated with increased sodium excretion
jTable includes comparison of most commonly found progesterones in the US oral contraceptive market. Progestational Activity: Levonorgestrel 5.3, Norgestimate 1.3, Norethindrone 1.0, Drospirenone 1.5. Progestational and androgenic activity are relative to 1 mg dose of norethindrone.
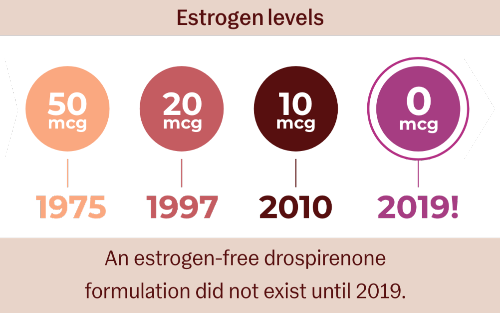
When the risk of estrogen is not worth taking,
Zero in on Slynd
- Like COCs, Slynd is effective with a familiar dosing regimen1
- Unlike COCs, estrogen-free Slynd is safe for women who cannot or do not want to take any unnecessary hormones1
Why are providers excited about Slynd?

Dr. Badeaux
Our office loves Slynd because it seems to work well in patients over 35 years old who smoke without carrying the estrogen-related riskl of potential increase in blood pressure and blood clots.
Use of Slynd (drospirenone) in patients with a history of thromboembolic disorders has not been evaluated in clinical trials. Discontinue Slynd if a thromboembolic event occurs.

Dr. Julie Mullins
A new patient came to me for her annual checkup; she was taking a combination birth control pill with 30mcg of estrogen. Given her smoking and hypertension, I suggested that she switch to Slynd. Now, over a year later, she’s still enjoying her experience with Slynd— highly effective contraceptive, low side effect profile, lighter periodsm. This is just one of many patient success stories I have had with Slynd!
Not all patients will have the same experience, some patients will experience breakthrough, irregular or no periods.

Dr. Alan Patterson
Slynd is my number one pill because my patients love it! They appreciate the effects of drospirenone and the adjustment period is just a few months for them to get to a manageable bleeding profile.k And, best of all, I don’t get callbacks!
In clinical studies, 3.5% of patients discontinued due to bleeding irregularities.
Resources
Office resources

Find out if Slynd® is a good choice for your patients
Talk to a sales rep today!
References
1. Slynd® package insert.
2. Data on file.
3. Micronor package insert.
4. Kimble T et al. Contraception:X. 2020:10020.
5. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
6. Muhn P, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Pharmacological characterization in animal models. Contraception. 1995;51:99-110. B.
7. Greer et al. Androgenic progestins in oral contraceptives and the risk of epithelial ovarian cancer. Obstet Gynecol. 2005;105:731-740.
8. Louw-du Toit R, et al. Biochem Biophys Res Commun. 2020; 526 (6):466-471.
9. Schindler AE, et al. Maturitas 2003;4651:S7-S16.
IMPORTANT SAFETY INFORMATION:
Indications: SLYND (drospirenone) tablets are a progestin, indicated for females of reproductive potential to prevent pregnancy.
Contraindications: SLYND is contraindicated in females with renal impairment, adrenal insufficiency, a presence or history of cervical cancer or progestin sensitive cancers, liver tumors (benign or malignant) or hepatic impairment, and undiagnosed abnormal uterine bleeding.
Warnings and Precautions
Hyperkalemia: SLYND has anti-mineralocorticoid activity, including the potential for hyperkalemia in high-risk females. Check serum potassium levels prior to starting treatment and during the first treatment cycle in females receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium concentration. Consider monitoring serum potassium concentration in females at increased risk for hyperkalemia i.e., those females who take a strong CYP3A4 inhibitor long-term and concomitantly with SLYND. Monitor females taking SLYND who later develop conditions and/or begin medication that put them at an increased risk for hyperkalemia.
Thromboembolic Disorders: Epidemiological studies have not indicated an association between progestin-only preparations and an increased risk of myocardial infarction, cerebral thromboembolism, or venous thromboembolism. Consider the increased risk of thromboembolism inherent in the postpartum period and in females with a history of thromboembolism. Discontinue SLYND if a thromboembolic event occurs and consider discontinuing SLYND in case of prolonged immobilization due to surgery or illness.
Bone Loss: Treatment with SLYND leads to decreased estradiol serum levels. It is unknown if this may cause a clinically relevant loss of bone mineral density.
Liver Disease: Discontinue SLYND if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and SLYND causation has been excluded.
Ectopic Pregnancy: Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on SLYND.
Risk of Hyperglycemia in Patients with Diabetes: Females with diabetes may be at greater risk of hyperglycemia and may require additional medication adjustments or monitoring. Progestins, including SLYND, may decrease insulin sensitivity.
Bleeding Irregularities and Amenorrhea: Females may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. If bleeding persists, occurs after previously regular cycles, or if scheduled bleeding does not occur, evaluate for possible causes such as pregnancy or malignancy.
Depression: Carefully observe females with a history of depression and discontinue SLYND if depression recurs to a serious degree. Data on the association of progestin-only contraceptive products with onset of depression and exacerbation of depression are limited.
The most common adverse reactions (>1%) are: acne, metrorrhagia, headache, breast pain, weight increased, dysmenorrhea, nausea, vaginal hemorrhage, decreased libido, breast tenderness, irregular menstruation.
Drug Interactions Drugs or herbal products that induce certain enzymes (for example, CYP3A4) may decrease the effectiveness of SLYND or increase breakthrough bleeding. Counsel patients to use a back- up or alternative non-hormonal method of contraception when enzyme inducers are used with SLYND and to continue back-up non-hormonal contraception for 28 days after discontinuing the enzyme inducer. Drugs or products that inhibit CYP3A4 may increase SLYND systemic exposure.
Please click here to access the full Prescribing Information.

Slynd® is a trademark of Exeltis Cenam
Boulevar Los Proceres
24-69, zona 10, ZONA PRADERA
Torre 3, Nivel 8, Of.801

